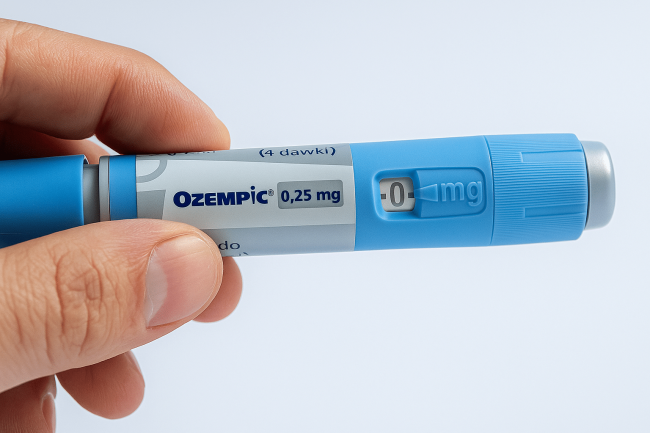
GLP-1 Agonists: Frequently Asked Questions
Curious about the science behind today’s most talked-about diabetes and weight-management treatments? Our new, four-part blog series on GLP-1 Agonists will break down everything you need to know — from how these drugs work, to their real-world results, side effects, and what’s coming next in the pipeline. Today, we’re taking a look at some of